CFDA Issued the Annual Report of Drug Inspection (2016)
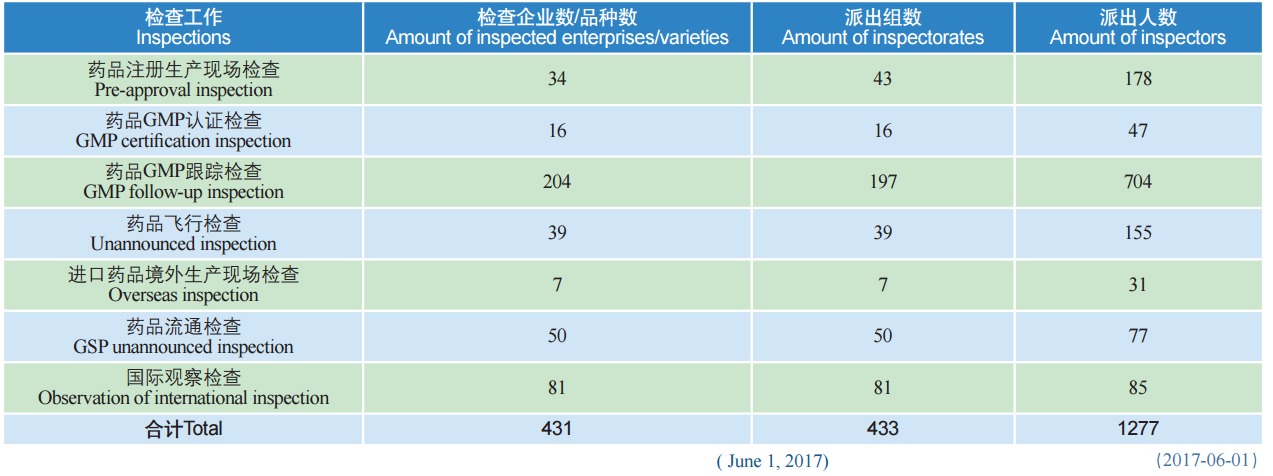
On May 31, 2017, CFDA issued the Annual Report of Drug Inspection (2016) in both Chinese and English versions, which elaborates the overview of inspections in 2016 and main findings therein, and analyzed the various bottlenecks and potential quality risks found in the inspections. The Report is divided into seven Parts, namely: Part I Pre-approval Inspection; Part II GMP Certification Inspection; Part III GMP Follow-up Inspection; Part IV Unannounced Inspection; Part V Overseas Inspection; Part VI GSP Unannounced Inspection; Part VII Observation of International Inspection.
Overview of the Inspections in 2016