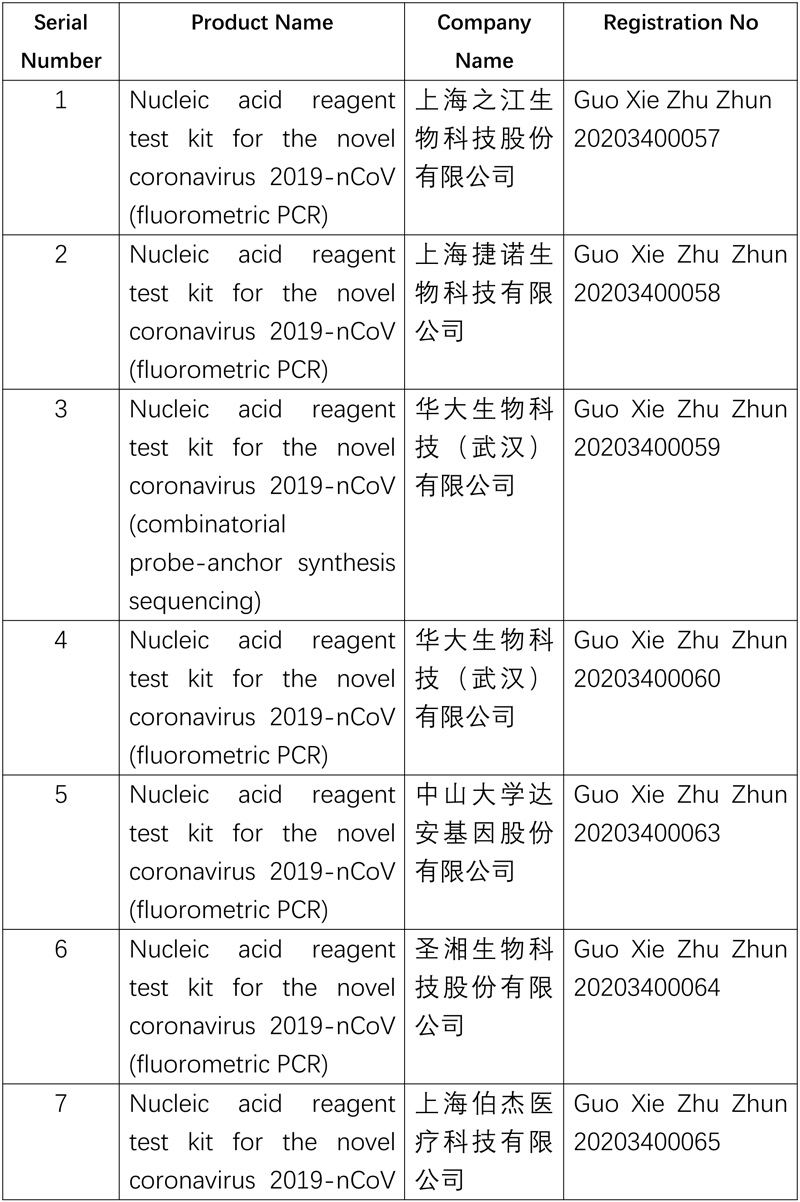
NMPA continues emergency approval of quick testing products for novel coronavirus
Updated: 2020-03-06
The National Medical Products Administration (NMPA) approved an antibody reagent test kit (chemiluminescence microparticle immunoassay) for 2019-nCoV developed by an enterprise on March 6.
The product uses an immunity analysis technology that combines paramagnetic nanoparticles and chemiluminescence. Based on the method of ELISA and with the support of an automated chemiluminescence device, the product can test for 2019-nCoV in human serum or blood plasma by detecting chemiluminescence signals. The approval of the product increases the supply of quick test kits and further serves epidemic prevention and control.
As of now, NMPA has approved ten nucleic acid reagent test kits and five antibody test kits for 2019-nCoV.