NMPA conducts emergency approval of testing products for 2019-nCoV
Updated: 2020-03-12
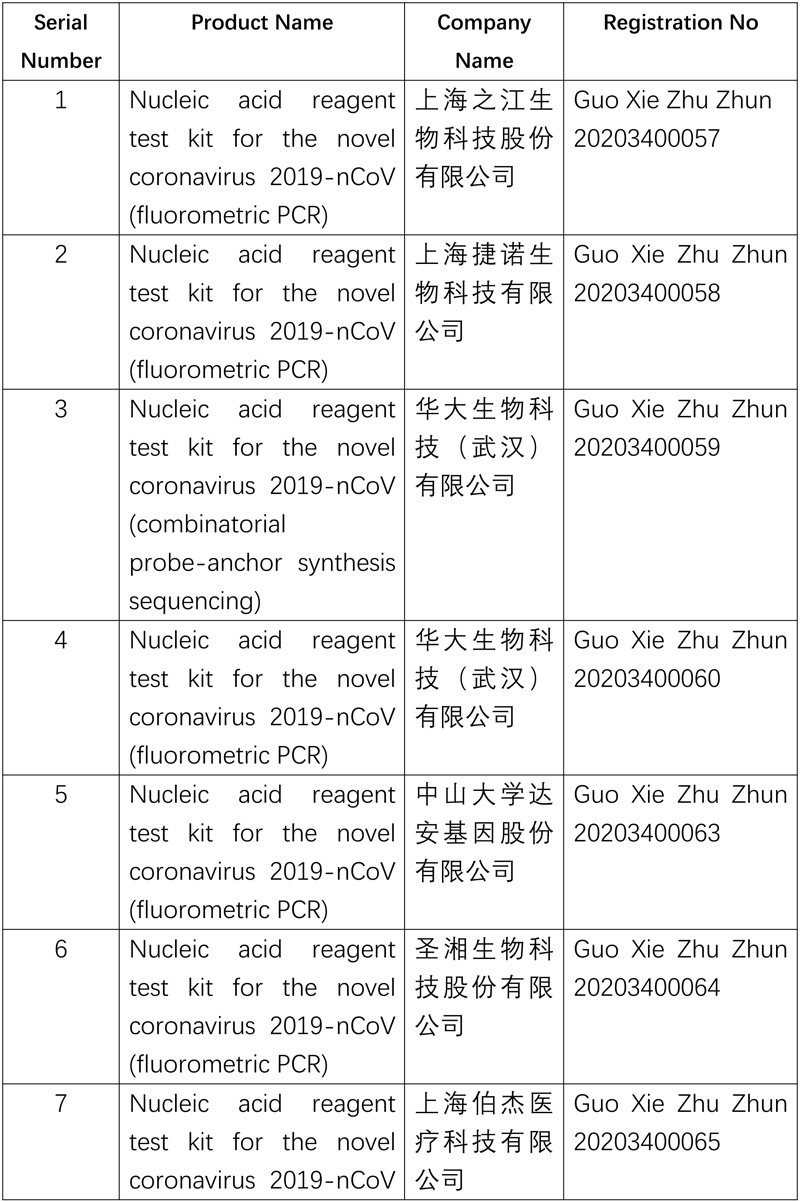
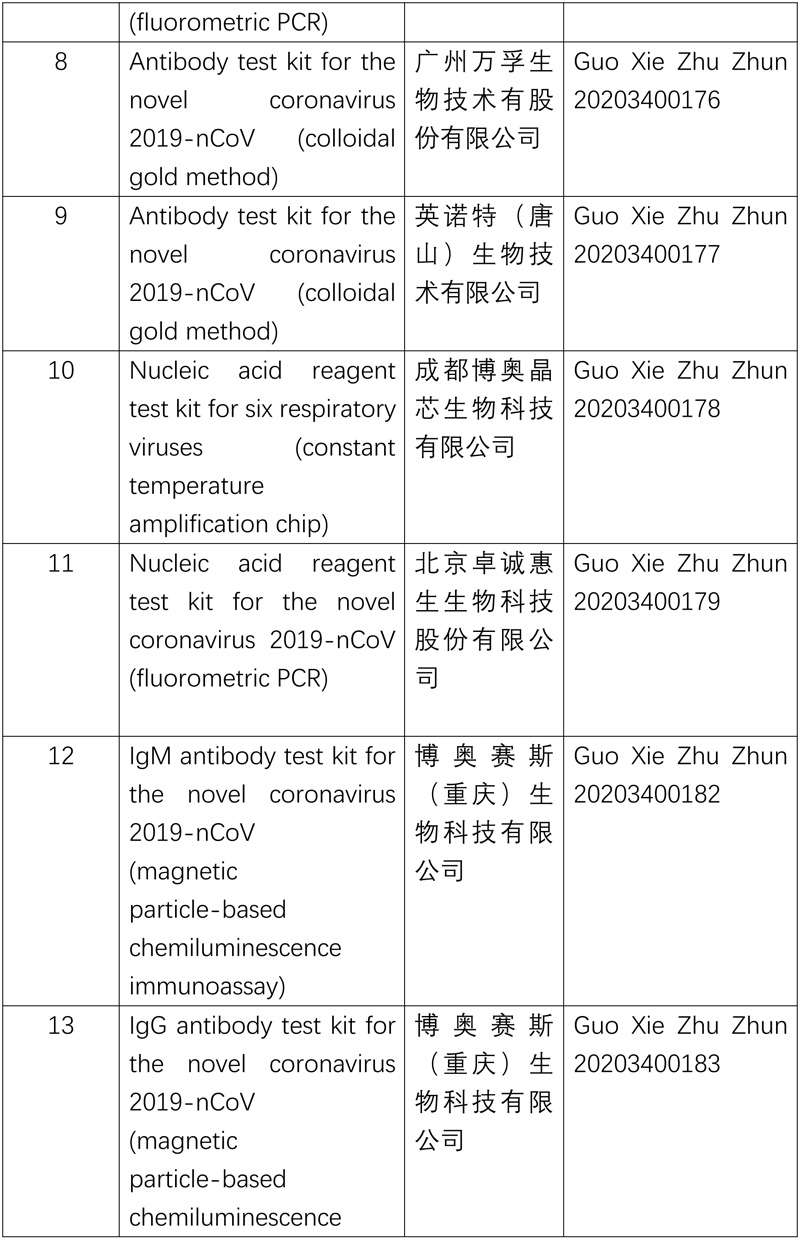
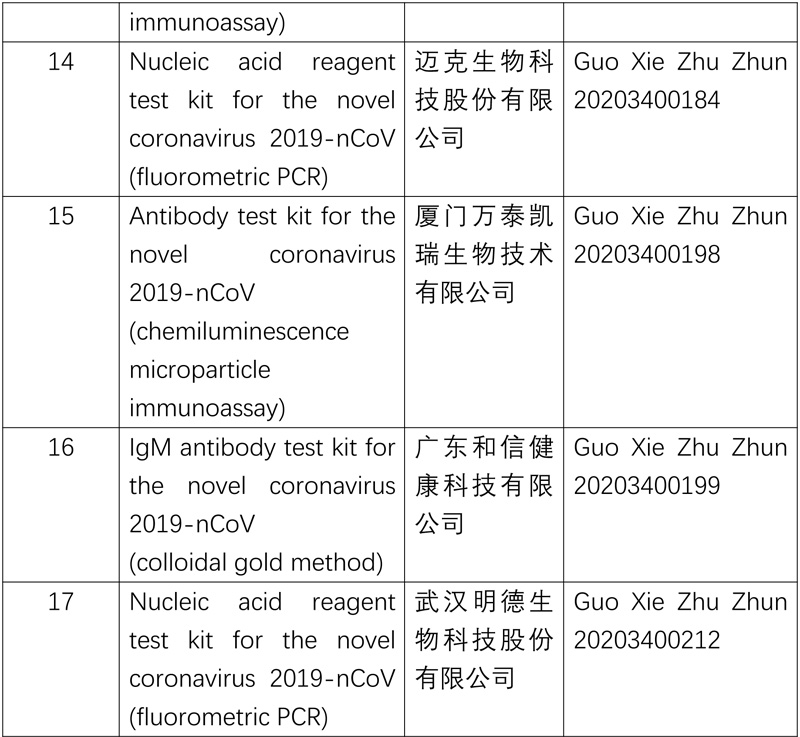
The National Medical Products Administration (NMPA) approved a nucleic acid reagent test kit (fluorometric PCR) product developed by an enterprise in Wuhan on Mar 12. Coupling the multiplex PCR-fluorometric probe testing method with the one-step RT-PCR technology, the product can diagnose patients with suspected 2019-nCOV-caused pneumonia, clusters of patients suspected of being infected with 2019-nCOV and others in need of 2019-nCOV testing by identifying the ORF1ad gene and N gene of the 2019-nCOV in throat swabs, nasopharyngeal swabs and sputum samples.
The approval of the product increases the supply of nucleic acid reagent test kits for 2019-nCoV and further meets the need of epidemic prevention and control.
So far, the NMPA has approved 11 nucleic acid reagent test kits and 6 antibody reagent test kits.