NMPA conducts emergency approval of quick testing products for COVID-19
Updated: 2020-03-11
The National Medical Products Administration (NMPA) approved an IgM anti-body reagent test kit (colloidal gold method)) for COVID-19 developed by an enterprise in Guangdong on March 11.
By adopting an immunity analysis technology, the product can test for the IgM antibody. The approval will increase the supply of quick test kits and further serve epidemic prevention and control.
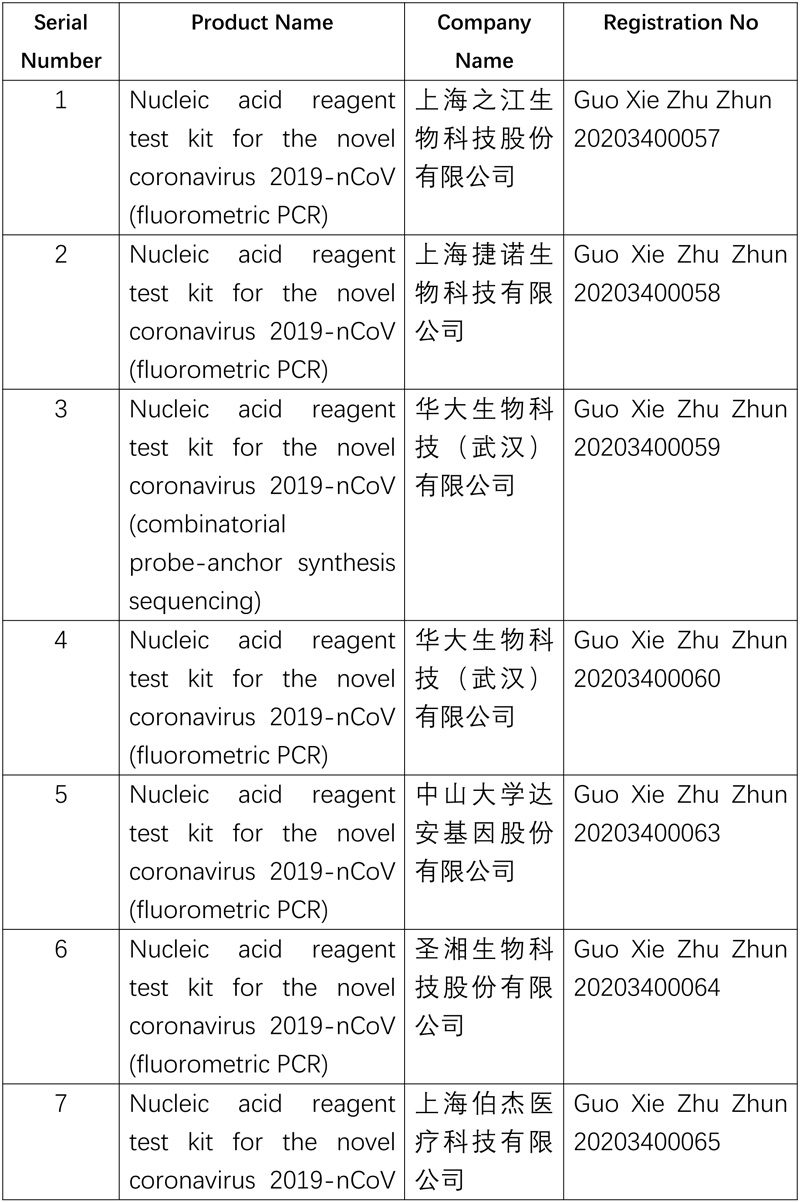
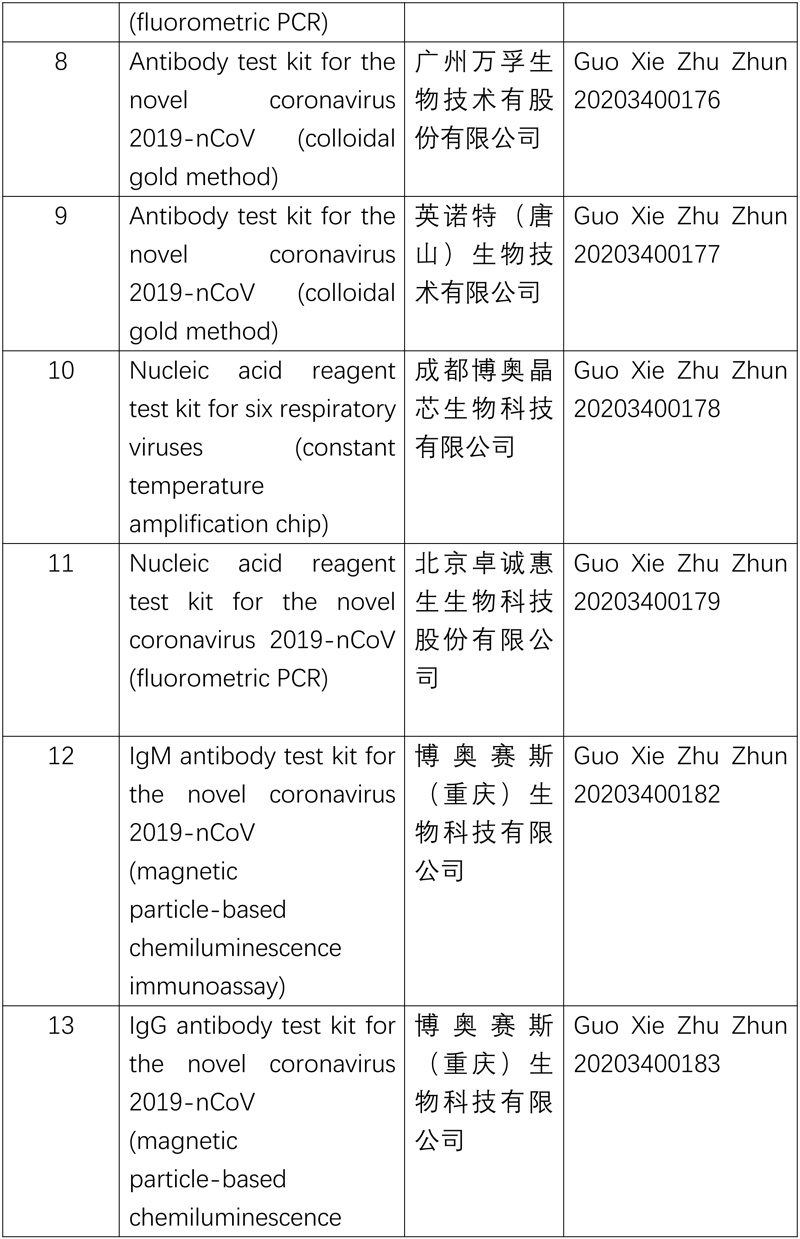
As of now, NMPA has approved ten nucleic acid reagent test kits and six anti-body ones for COVID-19.
The NMPA will continue the approval process for products needed to combat the pandemic.
Notes: Given the methodological characteristics of antibody test kits, the product can serve only as a supplementary test indicator for suspected cases with negative results from the nucleic acid test for COVID-19 or be used, in concert with the nucleic acid test, in the diagnosis of suspected cases. It cannot serve as a basis for confirming or ruling out pneumonia caused by COVID-19, nor can it be used in the screening of the general public. It can only be used by medical institutions.